american academy of pediatrics covid vaccine 12-15
The American Academy of Pediatrics AAP. Children and COVID Vaccine Data Report.
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org
Heres what the AAP says on kid vaccines.
. Learn more about the facts behind the COVID-19 Vaccine. ITASCA IL -- Today the Food and Drug Administration FDA finalized the full. The American Academy of Pediatrics recommends COVID-19 vaccines for all eligible children 5 years and olderMillions of kids and teens already have been safely vaccinatedVaccination.
ITASCA IL -- Today the Food and Drug Administration FDA. Presented by Sean OLeary MD MPH FAAP. The authorization was granted after Pfizer-BioNTech announced the vaccine was found to be 100 effective in preventing COVID-19 during a clinical trial of more than 2200.
Have been fully vaccinated. Adolescents moved a step closer to being able to receive a COVID-19 vaccine after officials on Monday approved Pfizer-BioNTechs vaccine for those ages 12-15 years. Thats over 13 million kids who have had both of their doses of COVID-19 vaccine.
Continues inoculating adults and adolescents questions remain about vaccinating the 48 million kids under the age of 12. The Science of Vaccines. Vice Chair Committee on Infectious Diseases.
CDC panel recommends Pfizers COVID-19 vaccine for kids as young as 12. The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents. MRNA Vaccine in Preventing SARS-CoV-2 Infection Among Children Aged.
Pfizer-BioNTech is in the process of testing its COVID-19 vaccine in children younger than 5 years down to age six months. Warren Seigel chair of the New York State chapter of the American Academy of Pediatrics is encouraging families to visit to their pediatricians ahead of school And as kids. The best way to slow the emergence of COVID variants is to get vaccinated.
Of vaccine for ages 12 to 15 as. Food and Drug Administration highlighting that the Delta variant has created a new and pressing risk to. The American Academy of Pediatrics AAP is urging the US.
American Academy of Pediatrics Cautions Against Off-Label Use of COVID-19 Vaccines in Children Under 12. With the delta variant raging almost five. The rollout of the PfizerBioNTech coronavirus vaccine for children ages 12 to 15 in the.
For the latest news on COVID-19 visit httpbitlyAAPNewsCOVID19. US urged to fast-track vaccine approval for children under 12 as cases rise Children accounted for 15 of new Covid cases reported last week American Academy of. Pfizer and BioNTech conducted trials in more than 2000 adolescents ages 12-15 with half randomized to receive the vaccine and half to receive a placebo.
Adolescents ages 12-15 years can begin receiving COVID-19 vaccine boosters. Ad Maximize protection get all recommended vaccine doses and boosters as soon as possible. Food and Drug Administration FDA to approve the use of COVID-19 vaccines in children younger than age.
It is anticipated that data will be submitted for FDA. Updated weekly this data report tracks the progress in vaccinating US children under 18 years of age. The American Academy of Pediatrics wrote a letter Thursday to the US.
A member of the American Academy of Pediatrics. AAP calls for completion of clinical trials of the vaccine in younger ages to identify correct dose. Ad FDA has authorized the Moderna COVID-19 Vaccine under an Emergency Use Authorization.
The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents. Learn more about the facts behind the COVID-19 Vaccine. Vaccines for children ages 5 and older.
Almost one-fourth of all kids 5. Over half of all kids 12 to 17 years old in the US. On May 12 2021 CDC approved the use of the Pfizer-BioNTech COVID-19 vaccine in persons aged 12-15 years following the vaccines EUA granted by the FDA on May 10th.
The American Academy of Pediatrics helped parents understand COVID with faster guidelines than the CDCs. As the US. State Level Data Report external icon American Academy of Pediatrics and the Childrens.
Ad FDA has authorized the Moderna COVID-19 Vaccine under an Emergency Use Authorization. The FDA has approved the Pfizer vaccine in teens 16 and older and granted emergency use authorization for the Pfizer vaccine for children 12 to 15. Adolescents ages 12 to 15 should get the Pfizer-BioNTech COVID-19 vaccine and they can get.

Why Experts Say It S Vital That Parents Get Their Kids Vaccinated For Covid

Children 12 15 Years Old Can Now Get The Pfizer Covid 19 Vaccine Uconn Today

Covid 19 Vaccine For Kids Benefits Are Many Here Are Answers To Parental Concerns Ask The Pediatrician Column Together Lancasteronline Com
Covid 19 Vaccine For Children And Adolescents Canadian Paediatric Society

Covid 19 Vaccines On Your Back To School Checklist Extension Monroe County

Covid 19 Vaccine For 12 To 15 Year Olds What Parents Should Know Methodist Health System Omaha Council Bluffs Fremont

Covid Vaccines In Teens And Heart Inflammation What You Need To Know Shots Health News Npr
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says

Faq Covid 19 Vaccine For Children Phoenix Children S Hospital

As School Resumes Uf Health Pediatrics Expert Gives Advice On Covid 19 Safety Uf Health University Of Florida Health
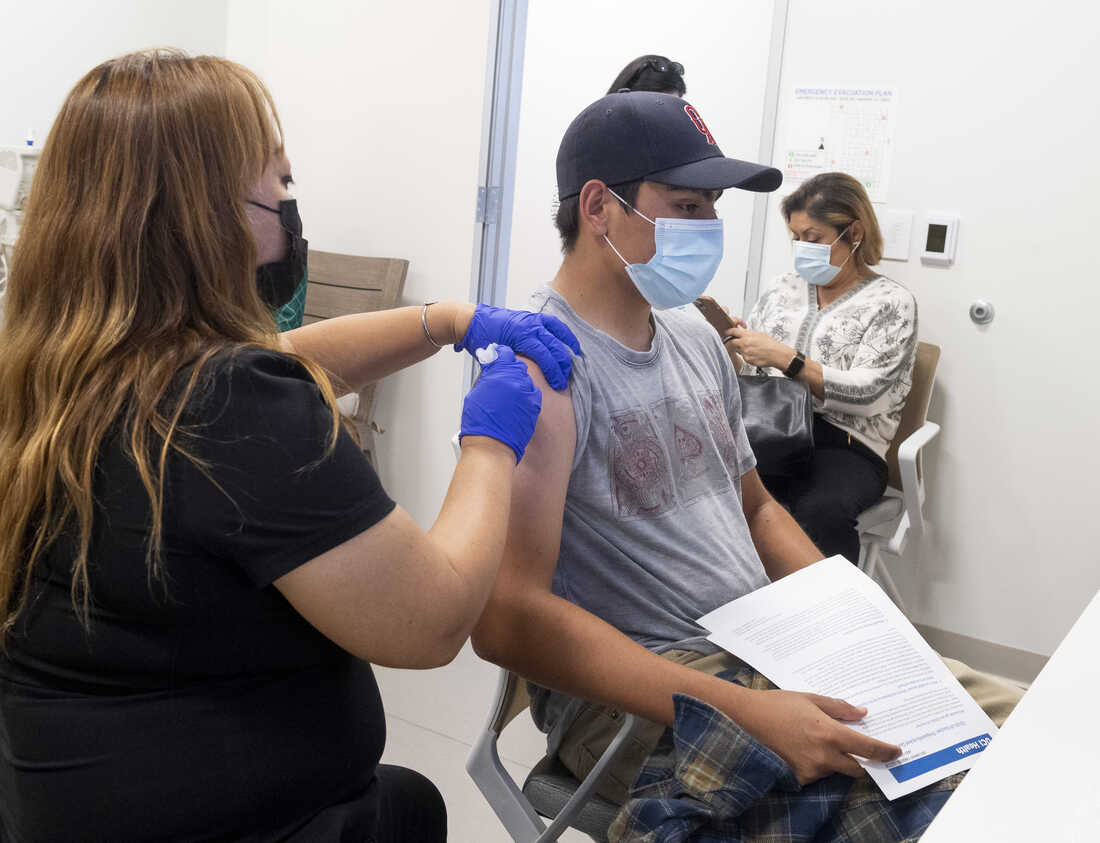
Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr

Cdc Committee Recommends That 12 To 15 Year Olds Receive The Pfizer Biontech Covid Vaccine

Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News
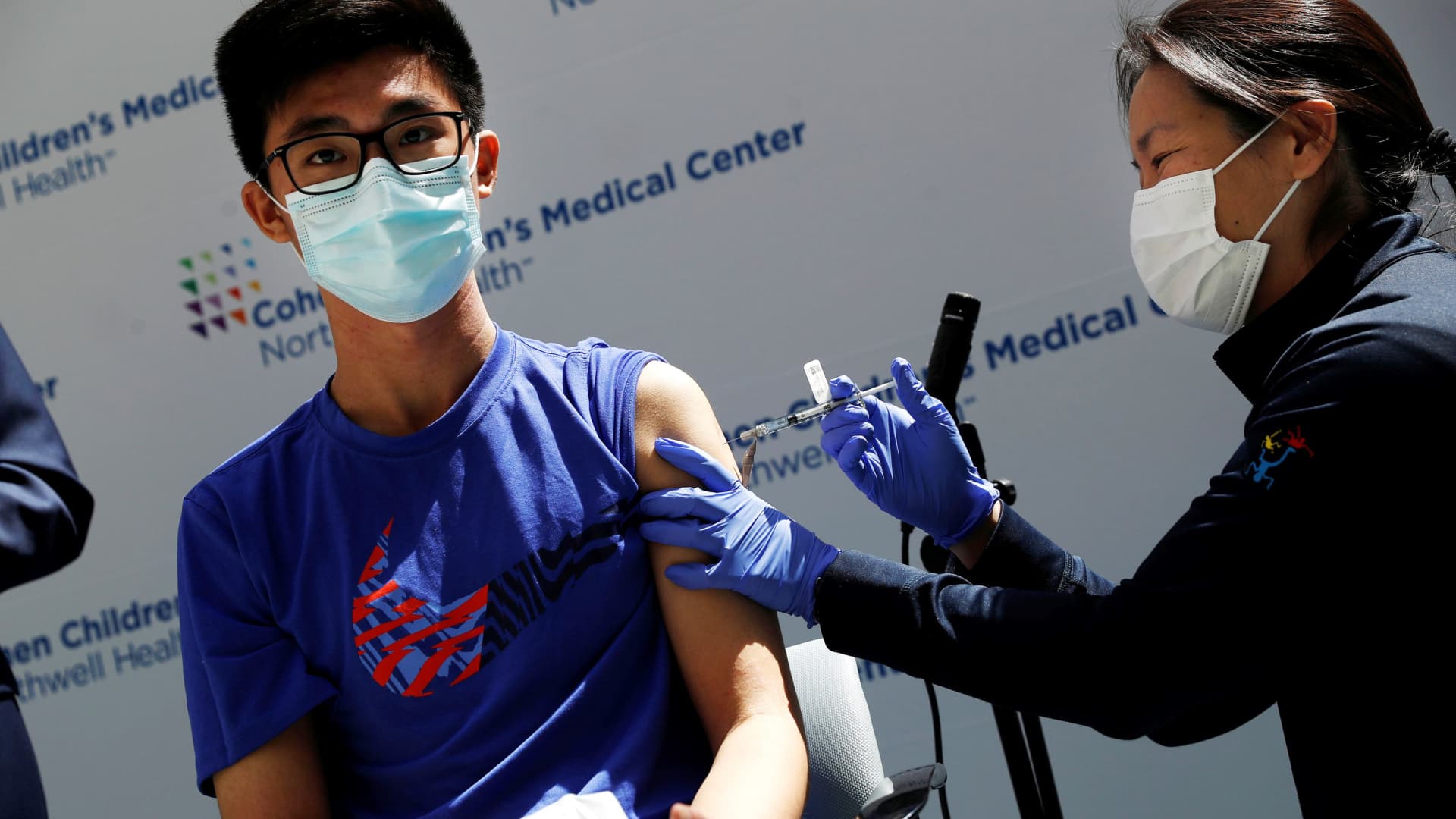
Back To School White House Pushes For Kids 12 And Up To Get Covid Vaccine

Kids Covid Vaccine Side Effects What To Know

Children As Young As 12 May Soon Be Able To Get Vaccinated
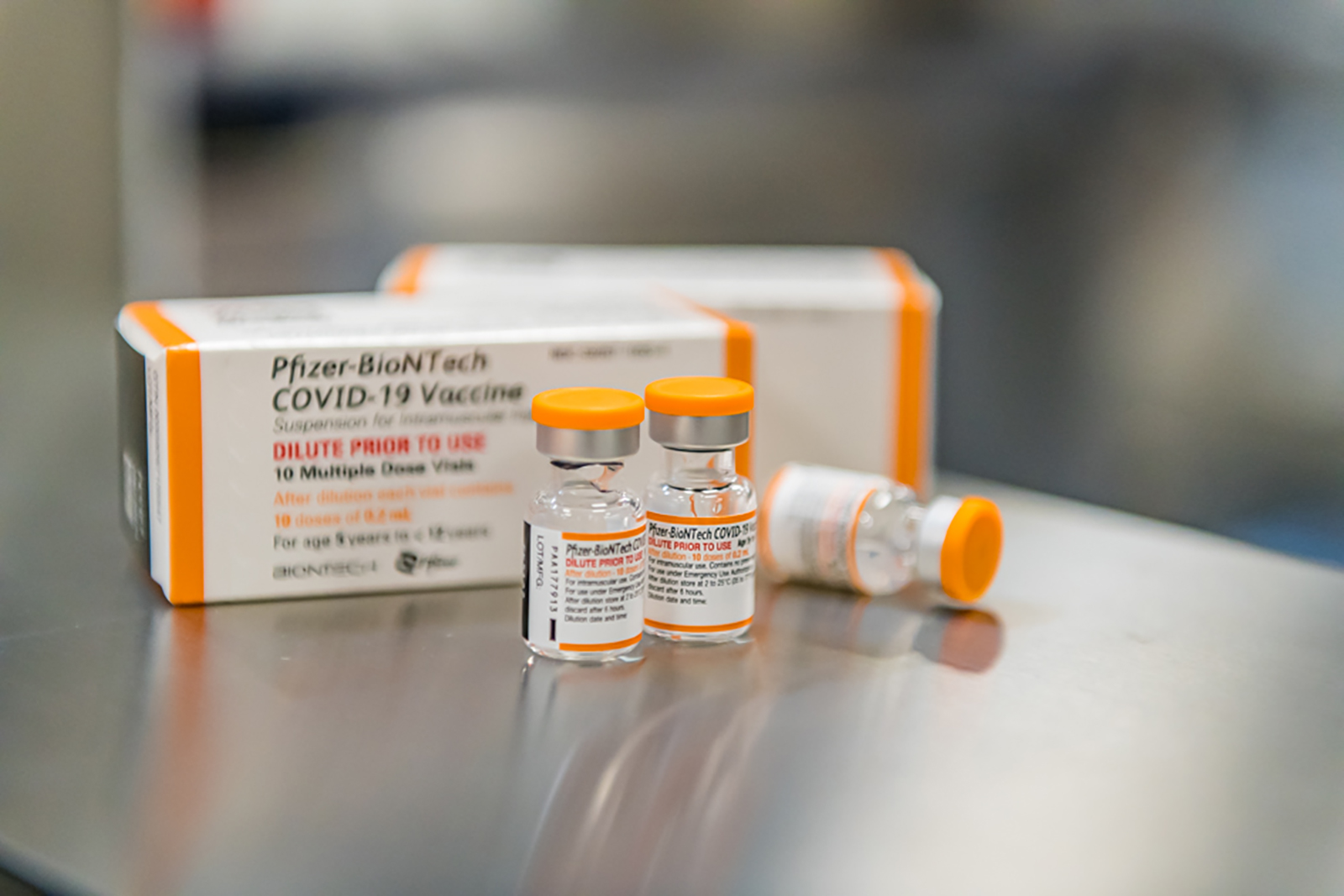
Younger Kids Could Be Getting A Covid 19 Vaccine Within Weeks Cnn
